
Über Hemostemix Inc.
Hemostemix (TSXV: HEM / WKN A2QQGU) wurde 2003 gegründet und ist ein führendes Unternehmen im Bereich der autologen Stammzelltherapie. Mit einer patentierten Plattform für blutbasierte Therapeutika fokussiert es sich auf die Entwicklung und Skalierung von Therapien für angiogene, neuronale und kardiomyozytäre Zellvorläufer.
Aktienkurs
Video-Präsentation
Aktuelle Nachrichten
Newsletter
Abonnieren Sie unseren Newsletter und bleiben Sie über die neuesten Entwicklungen, klinischen Meilensteine und Investitionsmöglichkeiten bei Hemostemix informiert.
Company Spotlight: Hemostemix Inc.
Hemostemix’s stem cell therapy works for no-option cases. Is it Your Fountain of Youth?
Your Fountain of Youth (TM)[1] may sound like science fiction, but it is not. Hemostemix (TSXV:HEM; FRA:2VF0) has treated heart patients, diabetic foot patients, vascular dementia patients, with scientific rigour and statistically significant results. Today, those who need stem cells to regenerate their circulation can obtain it. Soon, the Company predicts it will offer its therapy as naturally and regularly as one goes for an annual rejuvenation spa, enabling us to maintain sharp minds and a quality of life enjoyed in our 40s and 50s well into our 80s, and 90s.
Hemostemix has treated 498 individuals with complete safety and statistically significant results. It has published its scientific data of seven clinical trials of 318 subjects in the leading peer reviewed journals, including Stem Cell Research and Science. The company’s therapy is both safe and effective at regenerating circulation where the body signals that need. As with every medical innovation that becomes commonplace (think of weight loss pills), the need is great.
Think of the treatment of chest pain (Angina), a treatment for the heart after a heart attack, or the treatment of blocked circulation to the toes, feet and legs. Hemostemix has completed and published seven clinical trials that demonstrate it treats each of these conditions in patients who had no options. A no-option patient is typically at the end stage of their disease process. They are very ill, and they have exhausted all surgical, medical, and pharmaceutical options. Take, for example, a typical heart patient who has had stents, bypass surgery, and is maximally medicated and out of options. These are Hemostemix’s patients to-date.
The Hemostemix stem cell therapy is created from the patient’s blood. The Company is offering its treatment to eight groups of patients who have exhausted all surgical, medical, and pharmaceutical options; or, individuals who conclude: why wait until I am out of options? For example, Hemostemix treats patients facing limb amputation (no-option chronic limb threatening ischemia “CLTI”), peripheral arterial disease and chronic limb pain. In the heart patient population, it treats Angina, ischemic cardiomyopathy (plaque buildup), dilated cardiomyopathy (thickening of the heart wall), congestive heart failure, and vascular dementia, under special access programs.
Diabetics who become no-option CLTI patients face a 60% rate of mortality in five years. Hemostemix, in its Phase II trial, was noted by the University of Toronto and the University of British Columbia to save the limbs of 83% of patients followed for up to 4.5 years, who experienced healing of ulcers, cessation of pain, and zero mortality.
In its third study of the treatment of no-option heart disease, published in Stem Cell Research & Therapy, November 2023, Hemostemix confirmed the findings of two earlier independent studies: its therapy improved, by up to 27%, the percent of blood volume ejected with each heart beat in patients suffering from ischemic cardiomyopathy; and, improved the blood volume ejected with each heart beat by up to 47% in those patients suffering from dilated cardiomyopathy. These results were as statistically significant, as in its first study of 41 patients, and its second study of 106 patients.
With its proprietary stem cell therapy, Hemostemix is tackling these debilitating conditions earlier in the disease process, for revenue. In that regard, Hemostemix is no longer a start-up. It has been 21 years since the company was founded. Now, more than CAD $45.8 million has been invested, including more than $9.1 Million by management and directors since 2020.
Hemostemix was awarded the “Technology Pioneer Award” at the Davos World Economic Forum Summit in 2005, for proving scientifically that there are enough stem cells in 250 ml of the patient’s blood to develop a therapy from.
However, the road from a good idea to revenue realization is long and rocky. It speaks for the company that it is still delivering its therapy to the market after such a long time. Management has made substantial progress, has persevered, and has invested along with the Board of Directors approximately $9 million dollars since January 2020.
Clear path to Revenues ahead
During the Phase II clinical trial, the Company opted not to treat for revenue, to lower its risks. However, given its Phase II results, and given Hemostemix’s ability to treat no-option patients under special access programs, management negotiated a contract to manufacture its therapy at CytoImmune Therapeutics Inc.
Now, the company is focused on sales of its therapy to no-option patients and high net worth individuals who seek improved quality of life and longevity or improved cognitive abilities (vascular dementia). These treatments are being scheduled with specialist-physicians who have treated patients using Hemostemix’s therapy, including three cardiologists who treated more than 200 heart patients.

Figure 1: Hemostemix is ramping up production with immediate effect.
Starting with 20 batches per month, the company is aiming to double this to 40 batches per month by 2026. Simultaneously, the Company is developing it automated production system. Using robots, the company will simulate the production process, to enable it to scale to produce 240 treatments per month. At 20 treatments per month, the company forecasts to generate $8,800,000 per year.
Two types of stem cells
To understand the special position of Hemostemix, compared to its competitors, it is important to know that there are two main types of stem cells. There are stem cells that come from the patient’s body (autologous), and there are stem cells that come from the body of a donor (allogeneic). Intuitively, and scientifically, it is advantageous to use your own stem cells because they are completely safe, and more effective at leveraging the body’s natural healing processes. For example, Hemostemix’s therapy uses its GPS-like mechanism to navigate to the site of need, engraft, create new circulation, and recruit other stem cells to assist in the regeneration of the site of need.
The market for heart disease is estimated at $5.7 billion dollars and the critical limb ischemia market is estimated to be $3.5 billion dollars. Approximately 126 million people worldwide suffer from a heart disease known as ischemic cardiomyopathy. Approximately 236 million people worldwide have peripheral arterial disease, which affects blood flow to the toes, feet and legs. Approximately 23 million degenerate into CLTI, and approximately 5 million are no-option CLTI patients facing limb amputation.
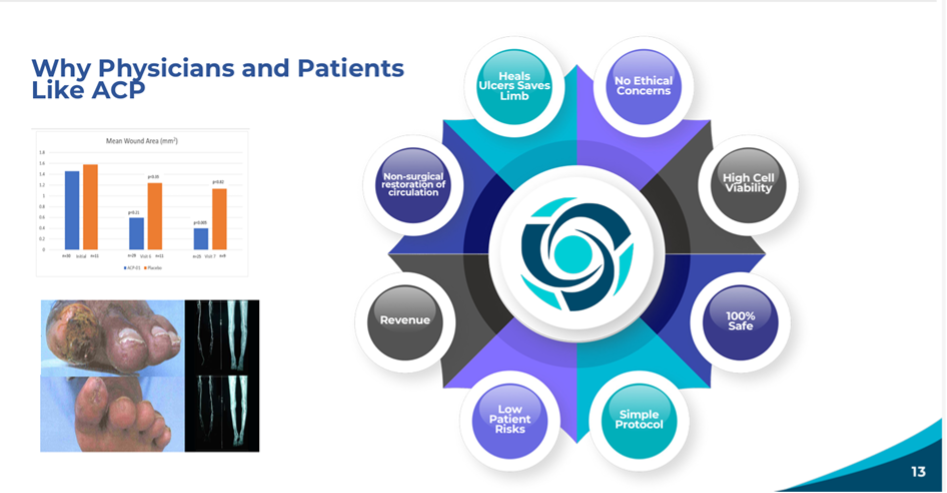
Figure 2: The image clearly shows how the precursor cells of blood vessels (ACP; Angiogenic Cell Precursors) go to the site of ischemia, where the body signals that it needs a new blood supply. This patient was saved from amputation. His toes healed.
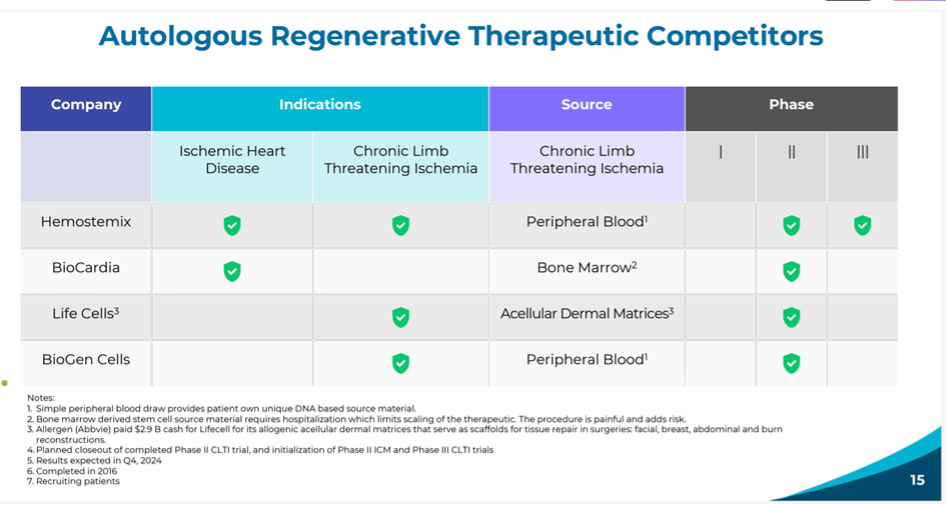
Figure 3: Overview of the competitors.
Strictly speaking, Hemostemix’s technology, blood vessel precursor cells (ACP-01, Angiogenic Cell Precursors) are only one of three products that Hemostemix can generate from the patient’s blood. Precursors of nerve cells (NCP-01; Neuronal Cell Precursors) and precursors of heart muscle cells (CCP-01; Cardiomyocyte Cell Precursors) are also generated from the patient’s blood. In this context, Hemostemix is a platform technology. NCP and CCP are not yet commercially available. However, the potential here is also significant: NCP may be ideally suited for a still young technology in which electrodes are implanted in the brains of quadriplegics. This approach has become particularly well known thanks to Neuralink (https://neuralink.com), an Elon Musk company. Hemostemix believes that NCP together with ACP will improve signal transmission between the brain and the electrode, and increase the longevity of the implant, which could reduce the need for additional operations.
The cardiomyocyte cell precursor is a heart cell precursor. Imagine your own heart stem cells beating in a dish. In the future, it may be fused with a bio scaffold to create a heart patch.
ACP is very easy for doctors to use, as it fits into their existing practice. For example, the nurse takes the patient’s blood on day one, and couriers it to Hemostemix, who processes it centrally. After the blood draw, the nurse schedules the patient to be in an appointment seven days later. The doctor receives ready-to-use syringes. If treating no option critical limb ischemia, the physician injects the therapy into the patient’s leg in an out patient procedure. If a heart treatment, the cardiologist and patient are in a catheter laboratory where the cardiologist injects the stem cells directly into the collateral arteries that supply the heart, a routine catheter procedure.
Building production from 20 to 240 batches per month
Hemostemix is now ready for phase III critical limb ischemia trial. The company plans to fund the study from the cashflow generated by the treatments of no-option patients.
The company plans for CytoImmune to produce 20 treatments per month with one team on one shift, to generate $8,800,000 revenue per year. By expanding to two or three shifts, revenue can double and triple. Thereafter, more significant volume (12X) is to be achieved by automating the production processes. Hemostemix expects to generate cash by Q4 2025 and be cash flow positive in 2026. To achieve this, Hemostemix will ramp up sales in the coming months.
Conclusion: Hemostemix is what is known on the stock market as a “fallen angel”. This is precisely why we also see the company as a bluesky trade. Hemostemix shares traded at the equivalent today of over CAD $20 when it went public in 2014! Today, the share price is in the penny stock range and the market capitalization reflects only a fraction of what has already been invested in the company.
The medical uncertainties are much lower today than they were a few years ago. Its clinical trials demonstrate that Hemostemix offers a breakthrough treatment for heart disease and chronic limb threatening ischemia.
There is no patient risk, as the therapy is the patient’s DNA. Therefore, there are no ethical concerns with the source of the stem cells. The therapy is safe and effective. The process is patented, scalable, and profitable. With the completion of a successful FDA Phase III clinical trial Hemostemix will become a very desirable takeover target.
We find Hemostemix an exciting biotechnology cardiovascular disease treatment investment. We will report on the company’s progress here from now on.
Disclaimer
GOLDINVEST Consulting GmbH offers editors, agencies and companies the opportunity publish comments, analyses and news on https://biotechinvest.de/. This content is intended solely for the information of readers and does not constitute a call to action; neither explicitly nor implicitly are they to be understood as a guarantee of possible price developments. Furthermore, they are in no way a substitute for individual expert investment advice and do not constitute an offer to sell the share(s) discussed or a solicitation to buy or sell securities. It is expressly not a financial analysis, but rather an advertising/journalistic text. Readers who make investment decisions or carry out transactions on the basis of the information provided here do so exclusively at their own risk. There is no contractual relationship between GOLDINVEST Consulting GmbH and its readers or the users of its offers, as our information relates only to the company and not to the reader’s investment decision. The acquisition of securities is associated with high risks, which can lead to a total loss of the capital invested. The information published by GOLDINVEST Consulting GmbH and its authors is based on careful research. Nevertheless, any liability for financial losses or the guarantee for the topicality, correctness, appropriateness and completeness of the articles offered here is expressly excluded. Please also note our terms of use.
According to §34b WpHG (Germany) and §48f Abs. 5 BörseG (Austria) we point out that GOLDINVEST Consulting GmbH, partners, authors, customers or employees of GOLDINVEST Consulting GmbH hold shares of Hemostemix Inc. and therefore a conflict of interest exists. GOLDINVEST Consulting GmbH also reserves the right to buy or sell shares in the company at any time. Under certain circumstances, this may influence the respective share price of the company.
GOLDINVEST Consulting GmbH is currently in a paid contractual relationship with the company, which is reported on the websites of GOLDINVEST Consulting GmbH as well as in social media, on partner sites or in e-mail messages. This is a clear conflict of interest. The above references to existing conflicts of interest apply to all types and forms of publication used by GOLDINVEST Consulting GmbH for publications about Hemostemix Inc. Furthermore, we cannot rule out the possibility that other market letters, media or research companies may discuss the stocks we cover during the same period. Therefore, there may be a symmetrical formation of information and opinions during this period. No guarantee can be given for the accuracy of the prices quoted in the publication.
BioTech Invest — 2025 All Right Reserved

